Most Atomic Solids Have Low Melting Points
True False Ionic compounds generally have low melting points. Quartz SiO2 is a solid with a melting point of 1550C.
10 5 The Solid State Of Matter Chemistry
Most ionic compouds are crystalline solids at room temperature.

. Cl2 atomic solid SiO2 ionic solid CaBr2 molecular solid. In atomic solids where the lattice points are occupied by Group 8 18 atoms the atoms are held together by _____ forces. As a chemist as a physical scientist you should look up some representative.
The bonding in quartz is best described as. What are the three categories we can divide solids into. Nonbonding atomic solids have low melting points.
True False When melted ionic compounds do not conduct electricity. True False The electrostatic attraction between an anion and a cation is an ionic bond. Most atomic solids have low melting points Selected Answer.
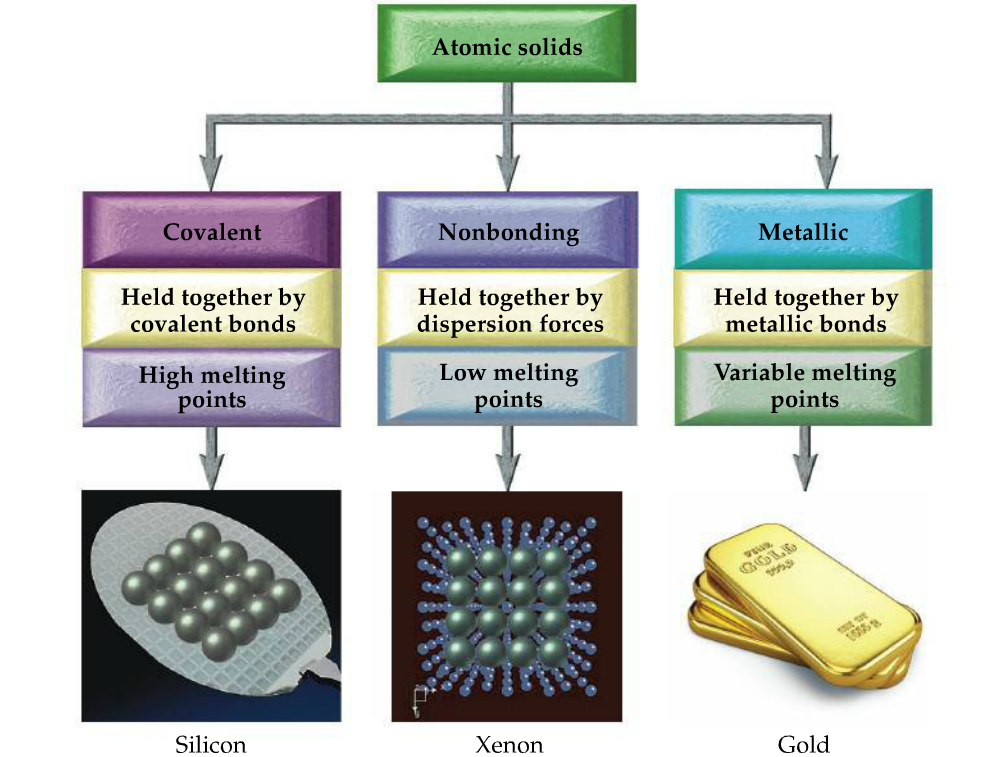
In cubic closest packing the unit cell is body-centered cubic. Covalent atomic solids non bonding atomic solids and metallic atomic solids. This attractive force is hard to disrupt and accounts for the high melting and boiling points of most ionic solids.
Atomic solids generally have low melting points. Metals which are physically flexible elements that can conduct heat and electricity tend to be solid at room temperature due to their relatively high. Examples of molecular solids with low melting and boiling temperatures include argon water naphthalene nicotine and caffeine see table below.
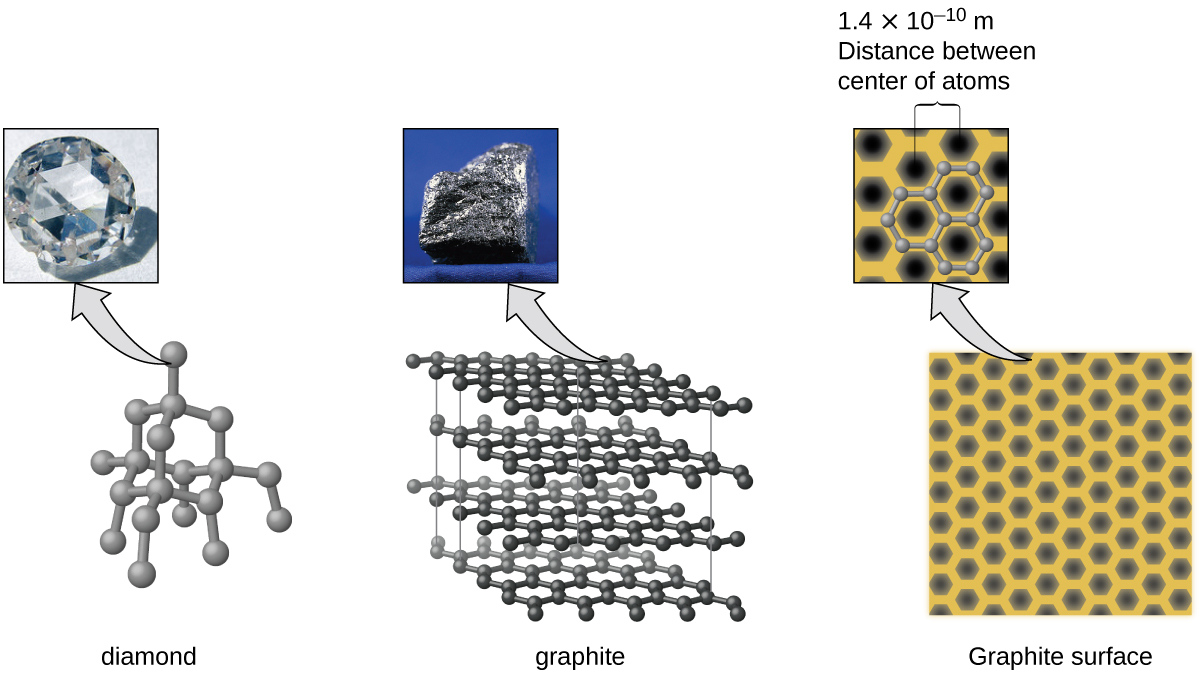
Nonbonding atomic solids such as solid xenon are held together by relatively weak dispersion forces. What are atomic solids. Here there are discrete molecules and typically intermolecular force is indifferent.
An atomic solid held together by dispersion forces. Most atomic solids have low melting points Selected Answer. SiO2 quartz form LiF Xe Fe.
For spheres of a given diameter tetrahedral holes are larger than octahedral holes. Much higher melting points than molecular solids. What are ionic solids.
This question was created from C9HW2pdf. Consequently solid xenon like other nonbonding atomic solids has a very low melting point about 112 C. What Lowers the Melting Point The melting point of an element is when it converts from solid form to a liquid.
Molecular solids have low melting T m and boiling T b points compared to metal iron ionic sodium chloride and covalent solids diamond. 122 cubic closest packing. Ionic solids tend to have.
Thus toluene C 6 H 5 CH 3 and m-xylene m-C 6 H 4 CH 3 2 have melting points of 95C and 48C respectively which are significantly lower than the melting point of the lighter but more symmetrical analog benzene. Ionic solids tend to have higher melting points than molecular solids. NaCl Na Cl2 SiO2.
Xenon atoms have stable electron configurations and therefore do not form covalent bonds with each other. Crystalline solid crystal Crystalline solids are orderly geometric structures in which atoms molecules or ions are arranged in patterns with long-range repeating order. Atomic solids generally have low melting points.
Moderately low Melting points. Based on your understanding of bonding in liquids and solids arrange the following substances from the highest to lowest melting points. Melting points of both metals and nonmetals vary widely but metals tend to melt at higher temperatures.
Contrast these properties with those of molecular solids. Self-healing rubber is an example of a molecular solid with the potential for significant commercial applications. Xe nonbonding atomic solids will have low melting points-however-noble gases have no interactions Which of the following is the most likely to have the lowest melting point.
Which of the following statements about the closest packing of spheres in binary ionic solids is false. True False Ionic compounds are electrically neutral. Solids who composite units are individual atoms.
15 Metals With The Lowest Melting Point Materials Science Engineering

Ionic Solids Molecular Solids Metallic Solids Network Covalent Solids Atomic Solids Youtube
Solved 3 Point Suppose A Sample Of Water Is Contaminated Chegg Com
No comments for "Most Atomic Solids Have Low Melting Points"
Post a Comment